12/30/2019
CMS Releases Long-awaited Details on MA/Hospice Carve-in Demonstration Planned for CY2021
On December 19, the Centers for Medicare & Medicaid Services (CMS) issued a Request for Applications (RFA) for the Value-Based Insurance Design (VBID) Model Incorporation of the Medicare Hospice Benefit into Medicare Advantage (MA), through which it is seeking participation by MA plans. Eligible MA plans in all states and territories may apply to participate in this component of the VBID model for CY2021. To date it is unclear which, if any of the MA plans active in Ohio will participate in the VBID.
Under the model, which is expected to run through CY2024, CMS will evaluate the impact on cost and quality of care for MA enrollees, including how the model improves quality and timely access to the hospice benefit, and the enabling of innovation through fostering partnerships between MA plans and hospice providers. To participate, plans will be required to offer palliative care services outside of the hospice benefit, as well as individualized transitional concurrent care, and will be permitted to offer additional supplemental benefits to support the seriously ill population. Participating plans will also be required to meet Wellness and Health Planning (WHP) requirements of VBID models generally, which include a systematic approach to advance care planning for enrollees with serious illness who are either not eligible for or who have chosen not to receive hospice services.
CMS expresses hope that, through improved coordination of care and inclusion of palliative and transitional concurrent care under the model, the median length of hospice stay will increase, very short and long lengths of stay will decrease, and enrollees and their families and caregivers will be able to experience the benefits of hospice care over a more appropriate period of time. As part of the RFA, CMS references concerns about spending outside of hospice under traditional Medicare; in addition to monitoring the model’s impact on hospice utilization patterns, CMS plans to monitor for any impact it may have on costs of care for treatments that are related and unrelated to the hospice terminal condition and related conditions.
The following represents an initial summary of the RFA.
In the RFA CMS outlined six main elements for the demonstration:
Participating plans must offer the full scope of hospice benefits. The hospice benefit under the plan is identical to that provided under traditional Medicare, and must be supplied under contract with Medicare-certified hospice programs. Plans are not permitted to “unbundle” hospice services and plans do not have the option of designing alternative ways of furnishing hospice services to enrollees who elect hospice. CMS expects participating MA plans to work collaboratively with in-network hospice providers to ensure coordination of care and that all necessary services are provided. Plans will be required to track service utilization and payment for Part A and B services and Part D drugs supplied to hospice enrollees outside the hospice benefit so that MA plans and CMS can determine whether hospice decisions that specific items or services were “unrelated” were appropriate.
Palliative care services. Plans will be required to have a strategy around access and delivery of palliative care services for enrollees with serious illness who are either not eligible for or who have chosen not to receive hospice services. These services are anticipated to be the types of palliative care services currently offered under Parts A and B of Medicare; must be budget neutral to total expenditures under Parts A and B; and must be either furnished, arranged for, or paid for by the MA plan. CMS describes these palliative care services as “largely medical services” covered under traditional Medicare; plans retain the right to separately provide stand-alone palliative nursing and social work services in the home that are not covered under traditional Parts A or B as a supplemental benefit (an expanded supplemental benefit option announced by CMS in April 2018). Plans will be required, as part of their applications, to delineate how care will be coordinated for enrollees, including how providers will develop an individualized plan of care inclusive of all relevant services and providers.
Transitional concurrent care services. To ease care transitions and ensure hospice-eligible beneficiaries are able to access and receive the full benefits of hospice care, participating plans must offer and work with in-network hospice providers and other providers to supply transitional concurrent care services necessary to address continuing care needs, as clinically appropriate, for the treatment of hospice enrollees’ terminal conditions. These services are intended to provide a less stark transition to hospice care, and may include a phasing out of specific curative treatments over time. Concurrent care will only be offered to patients that elect an in-network hospice provider. If the concurrent services are within the scope of an in-network hospice provider, it is possible that those services may be supplied by the hospice provider. During concurrent care, plans will be paid the capitated payment for Parts A and B, as well as the hospice capitated payment allowed under the model.
Participating plans will be permitted to offer a broad set of supplemental benefits for patients electing hospice care (in addition to mandatory supplemental benefits offered otherwise by the plan). These benefits could include:
- Coverage of primarily health-related services and items
- Coverage of non-primarily health related services and items to address social determinants of health that are expected to maintain or slow the progressive decline of health or the overall function of an enrollee based on socioeconomic status
- Coverage of non-primarily health related benefits of room and board within a hospice residential facility or equivalent residential facility
- Reductions in cost-sharing for services unrelated to a terminal/related condition during a hospice election
- Reductions in cost sharing for unrelated covered Part D drugs during a hospice election
“Care Transparency” for Beneficiaries, Families and Caregivers. To ensure transparency and improved beneficiary, family and caregiver experience with end-of-life care, CMS will monitor MA plans (participating plans and MA plans in the aggregate) on the following quality domains:
- Palliative care and goals of care experience
o Development of Advance Care Plans (ACPs) and Wellness and Health Care Planning (WHP)
o Access to, and use of, Palliative Care
o Proportion of Enrollees Admitted to Hospice for Less than 7 days
- Enrollee experience and care coordination at end of life
o Days Spent at Home in Last Six Months of Life
o Proportion Admitted to the Intensive Care Unit (ICU) in Last 30 Days of Life
- Hospice care quality and utilization
o Proportion of Lengths of Stay beyond 180 Days
o Transitions from Hospice Care, Followed by Death or Acute Care
o Visits in the Last Days of Life
o Experience of Care Measures (from Hospice CAHPS)
o Part D Duplicative Drug Utilization
o Unrelated Care Utilization
CMS anticipates making a quality payment adjustment in CY2023 for MA plans that participate during CY2021 and 2022 based on performance on a series of quality measures. At this time CMS plans to use the following measures, at a minimum, for purposes of the quality benchmark: (i) Proportion of Enrollees Admitted to Hospice for Less than 7 Days; (ii) Rate of Lengths of Stay beyond 180 Days; and (iii) Transitions from Hospice Care, Followed by Death or Acute Care. CMS may consider additional measures such as Days Spent at Home in the Last Six Months of Life and Proportion Admitted to the Intensive Care Unit (ICU) in the Last 30 Days of Life.
Network Requirements. CMS will phase in network adequacy requirements over the first two years of the model. CMS notes that plans have considerable discretion to select providers with whom to contract, but indicates that it expects plans to select hospice providers in a way that ensures that services are provided in a culturally-competent manner and accessible to all enrollees.
For CY2021 and 2022, a first year model participant must offer access to in-network hospices as well as out-of-network hospices, and an enrollee may secure hospice care from any allowed Medicare-certified hospice provider, except to the extent that the provider has been excluded by the plan based on concerns regarding risk of harm to patients. CMS indicates that participating plans may propose to exclude hospice providers that are found through publicly available data or sources to pose a risk for beneficiary harm; and/or consistently has been found to not offer the four levels of care, infrequently provided physician services, or rarely provided care on weekends. CMS is encouraging plans to implement a voluntary consultation process designed to increase enrollees understanding of their care choices (in and out of network).
For CY2022, plans participating for their second year may have a more formal version of the consultation program, which could include a requirement that enrollees electing hospice have a consultation prior to electing hospice with an out-of-network provider. For enrollees utilizing an out-of- network provider, hospice services must be paid at the traditional Medicare rates, but enrollees must be informed of their ineligibility for hospice supplemental benefits or concurrent care.
For CY2023 and future years, plans will be permitted to offer a hospice-specific point of service benefit for enrollees, but will be subject to a network adequacy requirement of having at least one Medicare-certified hospice that will provide access to services in the county and provide the full range of covered services.
Relative to cost-sharing, plans may not charge higher cost sharing than levels permitted under traditional Medicare (outpatient drugs and biologicals and inpatient respite). No other coinsurance or deductibles may be imposed for hospice services.
Payment Requirements. A participating MA plan cannot require prior authorization or implement other utilization management protocols that create inappropriate barriers to care. However, CMS will allow plans to implement program integrity safeguards in line with the plans policies and procedures. Examples provided by CMS are as follow:
- A prepayment review strategy to ensure that out-of-network hospice providers are providing drugs covered under the hospice benefit as necessary and that the cost of drugs covered under the benefit are not inappropriately shifted to Part D
- A prepayment review to address long lengths of stay (for example, greater than 180 days) to assess whether recertification was appropriate
CMS expects plans to impose timely submission requirements consistent with claims processing requirements under traditional Medicare. This includes timely filing of Notices of Election (NOE). CMS indicates it would expect late submission of NOEs to be accompanied by payment penalties.
For beneficiaries who elect hospice, MA plans will be paid as follows:
- A basic benefit capitation rate (the A/B capitation rate) will be paid for the month an enrollee elects hospice; this rate will not be paid for the subsequent month if, as of the first of that month, the enrollee still has elected hospice.
- For all calendar months that a beneficiary is on hospice care, the MA plan will be paid the following:
- A monthly hospice capitation rate established by CMS. The rate will reflect FFS paid hospice experience for care both related and unrelated to the terminal condition and related conditions (using data from 2016 through 2018 for CY2021 rate setting). The rate will not be risk adjusted based on beneficiary characteristics.
- A beneficiary rebate amount and
- The monthly prescription drug payment (if applicable)
For the first month of hospice care, the hospice monthly capitation rate will be adjusted depending on the length of time that a patient is on hospice care. CMS will establish a lower rate for lengths of less than 15 days, and a higher rate for lengths of stay on hospice that are 15 days or more.
CMS is expected to release additional information about the hospice capitation rate methodology in February. As part of the RFA, CMS outlines examples of payment arrangements that might emerge, including related to palliative care services outside of hospice, bundle payment for a serious illness care management program and hospice program, disease-state bundle payments, and sharing in decreased unrelated care costs.
The RFA outlines model requirements, including the types of plans that may participate, marketing and enrollee communications, how it will monitor the model and the data that it will collect, and general model oversight. Additionally, the RFA provides information on how it plans to evaluate the model, evaluation of the model and the application and selection process.
Following are the timeline and elements of the process CMS has identified for the model:

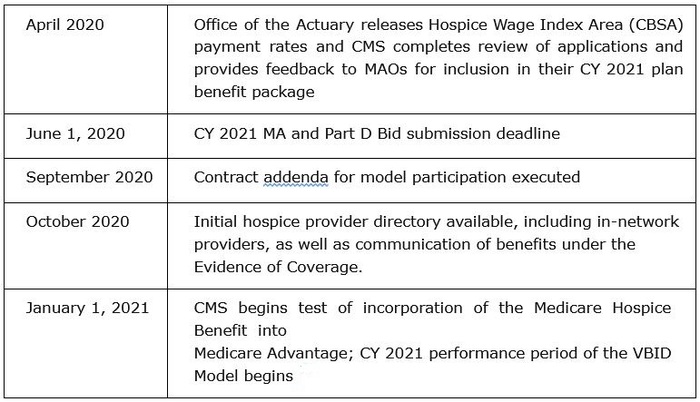
To date it is unclear which, if any of the MA plans active in Ohio will participate in the VBID. Questions regarding the VBID may be directed to Anne Shelley, Director of Regulatory Affairs for Home Health & Hospice, at ashelley@leadingageohio.org.
(shared from NAHC)
